
新入荷
再入荷
FDA Approves RECOVER IV Randomized Controlled Trial with Exception from Informed Consent (EFIC) | Business Wire
 タイムセール
タイムセール
終了まで
00
00
00
999円以上お買上げで送料無料(※)
999円以上お買上げで代引き手数料無料
999円以上お買上げで代引き手数料無料
通販と店舗では販売価格や税表示が異なる場合がございます。また店頭ではすでに品切れの場合もございます。予めご了承ください。
商品詳細情報
| 管理番号 |
新品 :264435374
中古 :264435374-1 |
メーカー | b4779b03b67 | 発売日 | 2025-09-02 11:34 | 定価 | 6300円 | ||
|---|---|---|---|---|---|---|---|---|---|
| カテゴリ | |||||||||
FDA Approves RECOVER IV Randomized Controlled Trial with Exception from Informed Consent (EFIC) | Business Wire
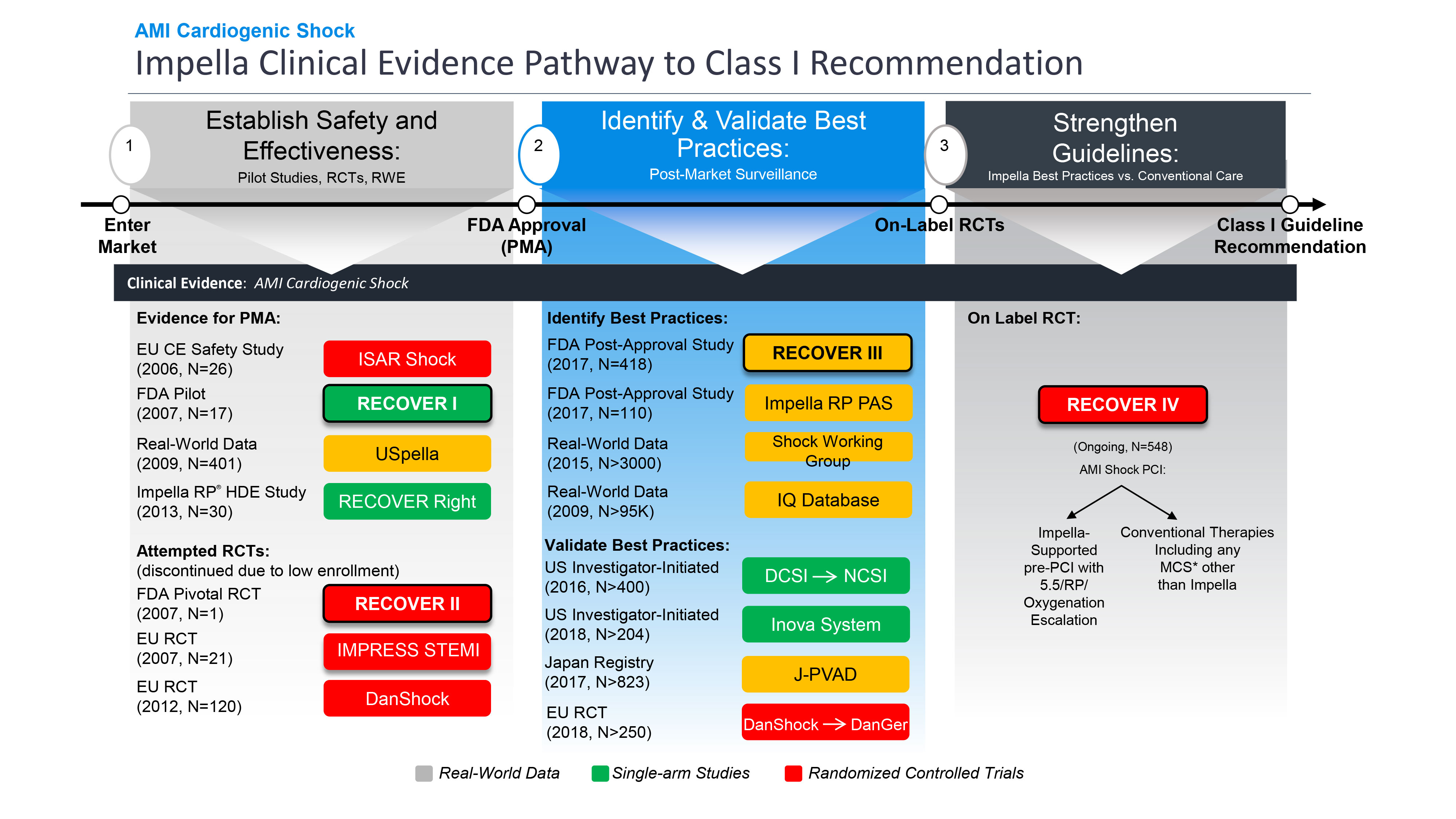 FDA Approves RECOVER IV Randomized Controlled Trial with Exception from Informed Consent (EFIC) | Business Wire,
FDA Approves RECOVER IV Randomized Controlled Trial with Exception from Informed Consent (EFIC) | Business Wire, Studies Led by NC State's Top Researcher Propelling First Drug to Treat Feline Heart Condition to Market | Veterinary Medicine News,
Studies Led by NC State's Top Researcher Propelling First Drug to Treat Feline Heart Condition to Market | Veterinary Medicine News, Personalized screening strategies for TP53 R337H carriers: a retrospective cohort study of tumor spectrum in Li-Fraumeni syndrome adult carriers - The Lancet Regional Health – Americas
Personalized screening strategies for TP53 R337H carriers: a retrospective cohort study of tumor spectrum in Li-Fraumeni syndrome adult carriers - The Lancet Regional Health – Americas



























